Introduction: Historically, CNS lymphomas have been associated with a very poor prognosis High-dose chemotherapy (HDC) with autologous stem cell transplantation (ASCT) has been investigated in patients with primary central nervous system lymphoma (PCNSL) and secondary central nervous system lymphoma (SCNSL) with promising results in small recent small prospective phase 1/2 results and retrospective series. We hypothesized that the use of R-BuMelTt (rituximab, busulfan, melphalan, thiotepa) conditioning regimen with ASCT regimen will be effective for both patients with PCNSL and SCNSL as used in previously reported promising data by Oh et al 2016 for patients with SCNSL with higher remission and survival rates.
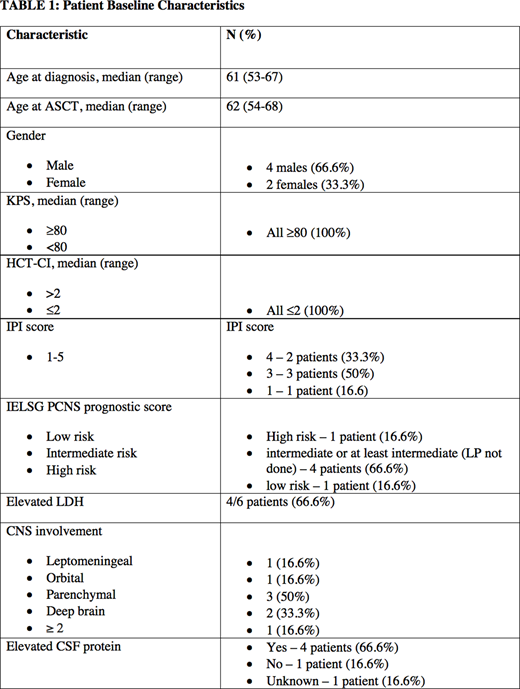
Patients and Methods: A retrospective analysis was performed of 6 consecutive patients who had undergone R-BuMelTt conditioning regimen with ASCT for 3 patients with PCNSL and 3 patients with SCNSL from December 2017 to March 2020. The median age of this patient population was 62 years at the time of ASCT. The induction chemotherapy regimen used for patients with SCNSL was R-CHOMP except one patient who received DHAP, and for patients with PCNSL MATRix regimen was used. The median duration of chemotherapy cycles was 4, and all of them had achieved remission prior to transplant based on PET/CT scan/MRI scan. All patients were planned to undergo ASCT using the Conditioning Regimen of Rituximab 375 mg/m2 on Day-7, Thiotepa 250 mg/m2 on Day-6,-5, Busulfan 3.2 mg/kg on Day-4,-3,-2 and Melphalan 100 mg/m2 on Day-1. Supportive cares measures were given at treating physician's discretion.
Results: Patients received a median cell dose of 4.4x106 CD34+cells/kg (range: 2.5-5.7), had neutrophil engraftment at 11.5 days (range:9-13), platelet recovery was achieved on days 11,15 and 16 for three patients but was delayed at 27, 46, 89 days for 3 patients. Infectious complications were common with documented bacteremia in in 3 out of 6 patients, 2 patients with c. difficile infection and with significant platelet support due to thrombocytopenia. At a median follow up of 24.5 months (range: 6-30 months), 5 out of 6 patients had complete metabolic response on radiological imaging with PET/CT in conjunction with MRI head. One of the patients with SCNSL died after transplant due to CNS relapse 223 days post ASCT giving an overall survival of 66.6%. Amongst the 2 patients with SCNSL that survived there was no relapse after 30 month follow-up. None of the patients with PCNSL died or relapsed during or after transplant therefore having a 100% overall survival. Although this is a small retrospective study, our results are comparable to current literature. Toxicities included nausea/vomiting, diarrhea, mucositis with 5/6 patients requiring TPN. Also busulfan PK levels were not done.
Conclusions: R-BuMelTt regimen can be used successfully as conditioning regimen for ASCT for patients with PCNSL and SCNSL, however with increased hematological toxicity, most notably delayed platelet engraftment. The rate of progression free and overall survival is promising with short median follow up of 24 months.
Lam:Janssen: Honoraria, Speakers Bureau; Roche: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal